About Zeesan
Company Overview in Numbers






Mission
Advance molecular diagnostic tools to unlock the valuable medical insights encoded in the basic building blocks of human body – nucleic acids, making a difference in improving early detection of diseases, precise treatment and prognosis monitoring.

Vision
We are ambitious to bring our unique and reliable solutions to clinical healthcare from all over the world, working towards a future where everyone has access to timely diagnosis for prevention and life-changing treatment.
Our Technology
Multicolor
multiple probes
Melting
melt curve analysis
Curve
each mutation has its Tm
Analysis
single step like
real-time PCR
*MMCA-based multplex PCR platform has been granted several International invention patents.
Patented Multicolor Melting Curve Analysis (MMCA®) overcomes the limitations of conventional RT-PCR in terms of the maximum number of detectable targets (usually between 2-5, depending on the number of fluorescence channels) in one tube.
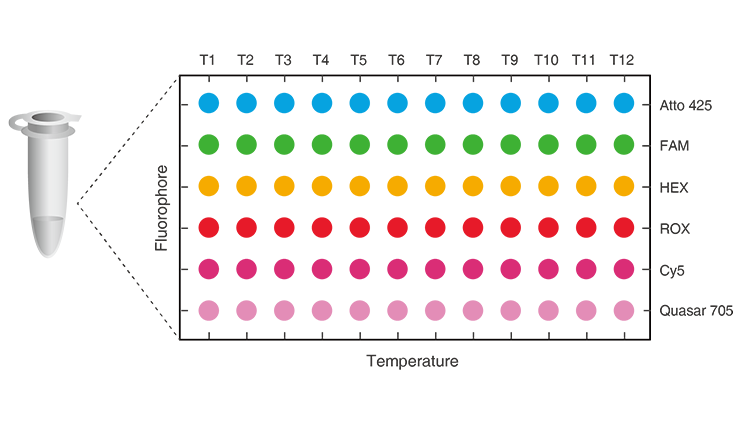
*Huang et al, PNAS, 2022
MMCA 2D Label = Fluorescence Channels + Melting Curve
12 targets/channel X 6 fluorescence channels
With MMCA®, up to 72 targets can be detected simultaneously in one tube. As short as 40 minutes to result
Our History
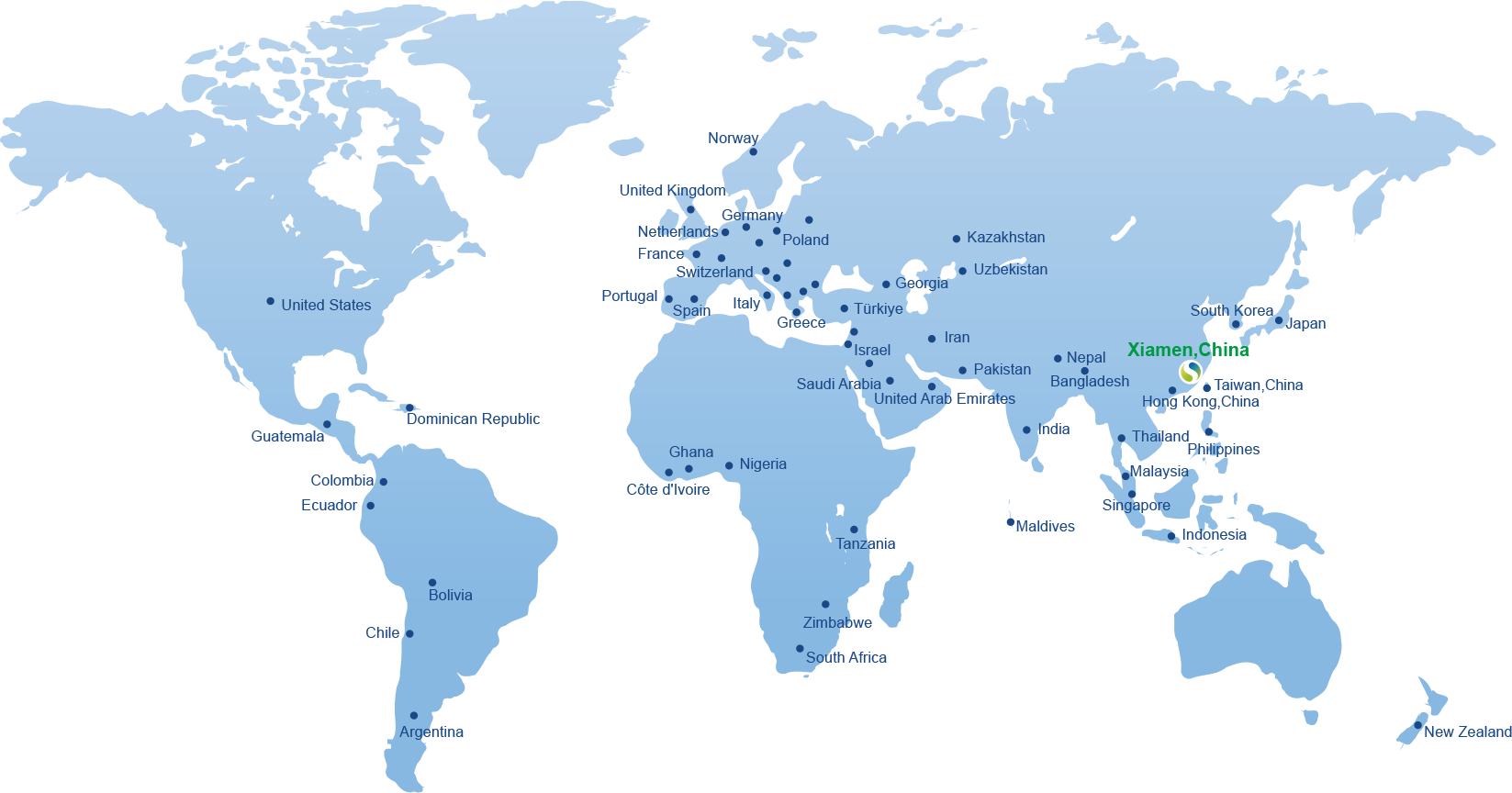
Our Global Partners
Interested to Know More about Our Solution to Molecular Diagnostics?